Modelo del impacto del tratamiento farmacológico y no farmacológico para la enfermedad de Alzheimer
Yanliel Feliciano González
Departamento de Ciencias Naturales
Facultad de Ciencias Naturales, UPR Cayey
Nanelli Feliciano González
Departamento de Ciencias Naturales
Facultad de Ciencias Naturales, UPR Cayey
Recibido: 18/09/2025; Revisado: 12/11/2025; Aceptado: 18/11/2025
Abstract
Alzheimer's is a progressive neurodegenerative disease that impairs cognition and memory. Our research studies the mechanisms of AD and evaluates the effect of pharmacological and non-pharmacological treatments, using differential equation simulations. We modeled galantamine, an acetylcholinesterase inhibitor that increases acetylcholine to improve cognitive function, and lecanemab, an antibody that reduces Beta-amyloid accumulation. Non-pharmacological treatment was modeled as physiotherapy interventions designed to maintain cognitive function through increased physical activity. Combined treatments demonstrated a synergistic effect, maintaining higher cognitive levels and reducing biomarker accumulation more effectively than single therapies. This research highlights the potential of computational models to explore interventions for Alzheimer's.
Keywords: lecanemab, galantamine, cognitive, beta amyloid, differential equations
Resumen
El Alzheimer es un trastorno neurodegenerativo que afecta la cognición y memoria. Este estudio investiga los mecanismos de la EA, evaluando tratamientos farmacológicos y no-farmacológicos, mediante la simulación de ecuaciones diferenciales. Se estudiaron tratamientos como la galantamina, un inhibidor de acetilcolinesterasa, y el lecanemab, un anticuerpo que afecta el EA, así como tratamientos no farmacológicos, como la fisioterapia, durante la progresión del Alzheimer. La combinación de tratamientos mostró un efecto beneficioso, manteniendo el rendimiento cognitivo y una menor acumulación de biomarcadores en comparación con los tratamientos individualizados. Esta investigación sugiere que estrategias terapéuticas y modelos computacionales pueden ser herramientas para explorar intervenciones contra el Alzheimer.
Palabras clave: lecanemab, galantamina, cognitivo, betas-amiloides, ecuaciones diferenciales
Introduction
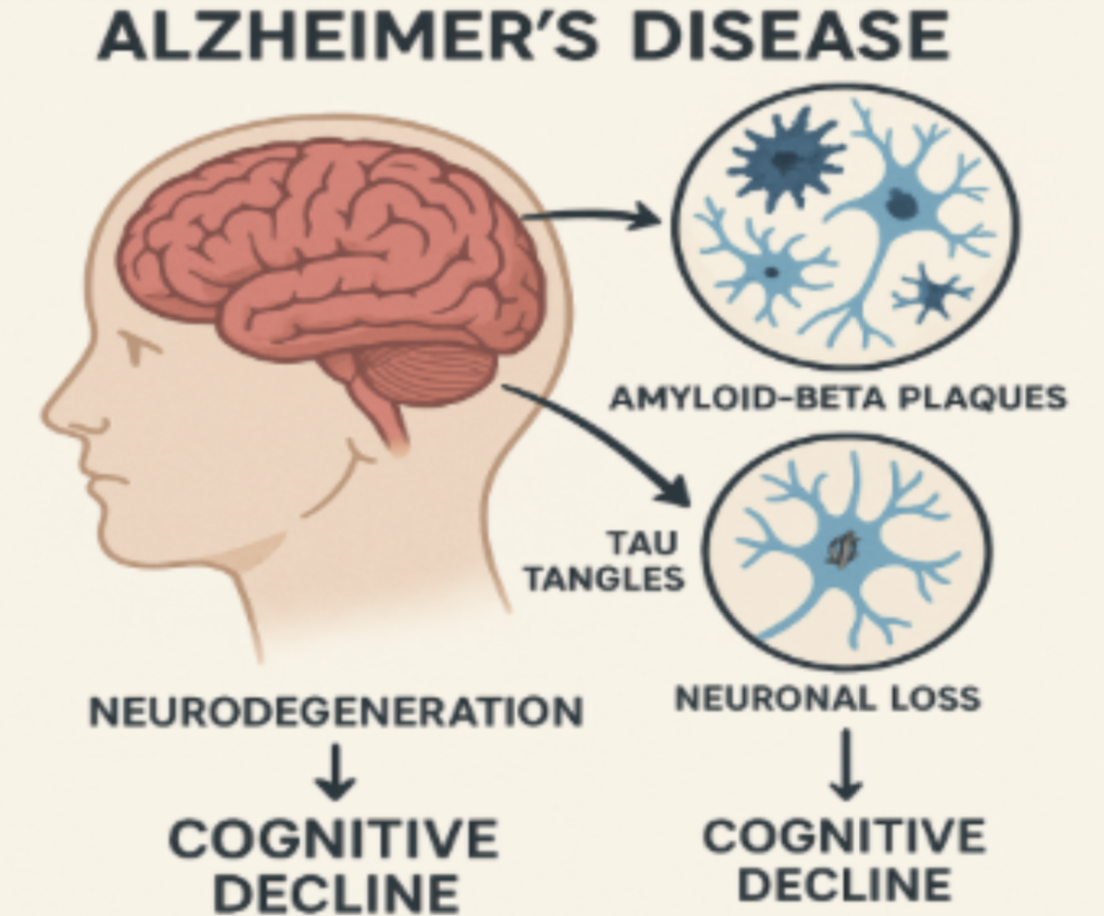
Alzheimer's disease (AD), characterized by neurodegeneration and cognitive impairment, affects millions of people in the world, causing a gradual loss of theirmotor functions and memory. Characterized by the accumulation of beta-amyloid plaques and the Tau protein (Figure 1), Alzheimer's represents one of the main causes of dementia, which leads to greater care by health personnel and their families (Hao et al., 2022). Worldwide, 55 million people have dementia, of whom between 60% to 70% have AD. Among these 70% are 75 years or older (Mayo Clinic, n.d.). Alzheimer’s and other dementias impact Puerto Rico with 54,473 cases registered between 2008 and 2024, which is 68.6 percent corresponding to AD. This condition has been consolidated as the fourth cause of death, climbing to become the third cause of death for women over 60 years old since 2018 (Departamento de Salud de Puerto Rico, n.d.). In the USA, 7.2 million people aged 65+ live with AD, which is 11% of the population; this is expected to increase to 13 million by 2050 (Alzheimer’s Association, n.d.). It is important to emphasize that Alzheimer's has no cure, but some treatments help slow down the progression.
Figure 1: Alzheimer’s biology, Copilot, 2024
Source: Image generated with Artificial Intelligence
Currently, there is a neurotransmitter that can treat the condition of Alzheimer's and help to improve cognitive function and memory. It is an acetylcholinesteraseinhibitor called Galantamine. Although its effectiveness has been demonstrated over time, its impact on the patient's disease will be limited over time. This is because galantamine increases acetylcholine levels, temporarily improving memory and cognitive function (Georgieva et al., 2023; Raskind et al., 2004). However,as the disease progresses, the neurons responsible for producing and using acetylcholine continue to degenerate, reducing the drug's response. This has prompted the search for complementary therapies capable of addressing the multiple aspects of Alzheimer’s pathology (Raskind et al., 2004).
In response to this need, non-pharmacological interventions have been fundamental in this process. Among them, physical exercise has been studied as a promising therapeutic strategy in AD (Chen et al., 2016). Studies have shown that physical activity mitigates or slows cognitive decline, which, in turn, reduces Beta-amyloid plaques and improves cognitive function by increasing blood flow through increased blood flow from constant movement (Afsar et al., 2023; Chen et. al.,2016; Delgado-Peraza et al., 2023).
To understand and predict the impact of combined interventions, it is imperative to use tools that simulate the interactions between treatment effects and biologicalfactors. For this reason, mathematical models are a tool for analyzing the progression of AD, enabling us to systematically evaluate the effectiveness of strategies.
The present study aims to investigate the combined impact of the intervention of pharmacological and non-pharmacological treatments on the progression of Alzheimer's disease. Through the development and application of the mathematical model, the following are sought:
To investigate the mechanisms of Alzheimer's and how pharmacological treatments impact the progression of the disease.
The aim is to study how it can help the patient to delay the progression of Alzheimer's.
Seek new insights into the mechanisms of neurodegeneration.
Throughout this investigation, we will present the mathematical model composed of differential equations, conduct simulations, highlight the importance of treatment combinations for disease management, and discuss our findings.
Methodology
The methodology of this research was based on a theoretical and computational approach to study the progression of Alzheimer's disease through the literature review, focusing on simulations to study its progression. A mathematical model was developed based on the model presented by Hao et al. (2022) to understand the disease's behavior, using data and findings from the scientific literature. The research was divided into three main methods: literature review, mathematical study and development, and computational analysis.
One method used to determine which medications affect or delay the progression of Alzheimer's in the early stages was to review scientific articles evaluating their relationship with our study approach. From scientific articles, we identify equations that establish relationships among components affected by AD (Hao et al., 2022). In this study, we used a cascade model that incorporates four clinical biomarkers of Alzheimer’s. They are Beta-amyloid, Tau, neuronal loss, and cognitive impairment. The physiological duck network of Alzheimer’s, as briefly illustrated in Figure 1, begins with the presence of Beta-amyloid in plaques, which favors the abnormal phosphorylation of the tau protein, leading to neurodegeneration and, ultimately, brain alterations, resulting in cognitive impairment (Hao et al., 2022).
System of ordinary differential equations
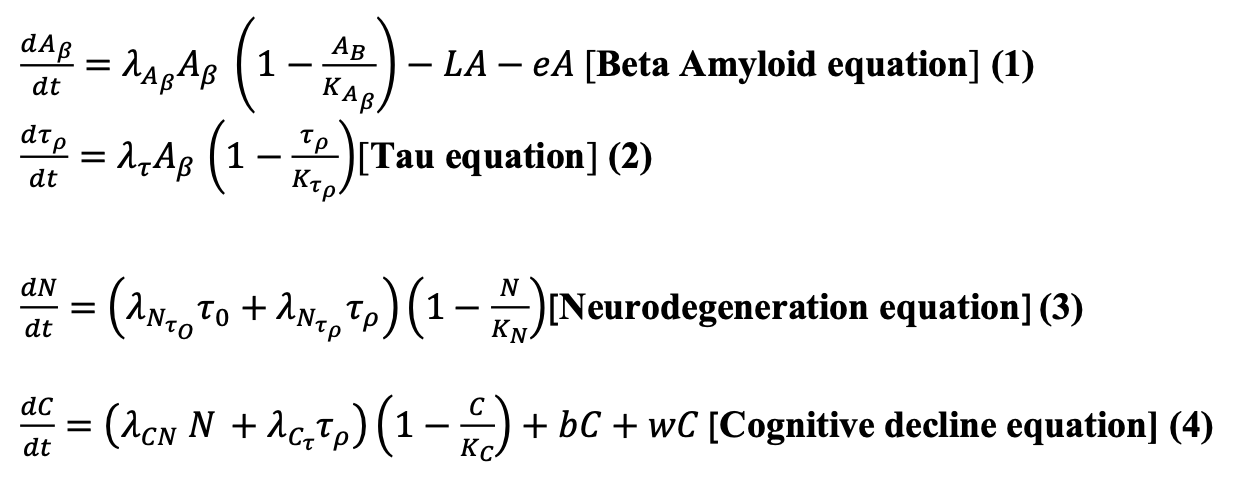
To study the mechanisms of AD and simulate the effects of pharmacological and non-pharmacological treatments, we use the differential equation system presented by Hao et al. (2022) and add terms to incorporate the effects of these treatments we want to explore in our simulations.
The system of differential equations is as follows:
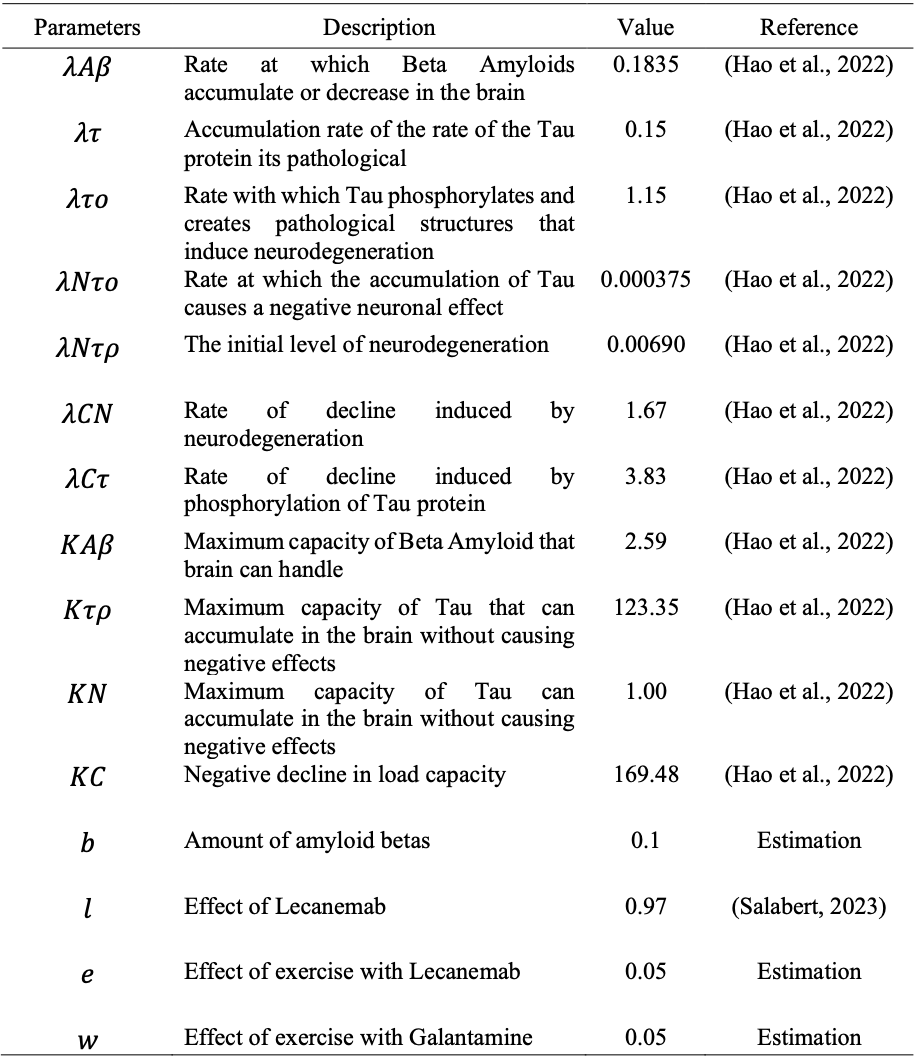
The Beta-amyloid equation (1) represents the key event in the neurodegenerative condition. It is estimated to arise from an imbalance in the production and elimination of AB, which leads to the accumulation of amyloid plaques. The logistic growth equation models the AB. In this original equation by Hao et al. (2022),we added two negative terms, 𝐿𝐴 and eA, represented as the effect of the treatment of Lecanemb on amyloid minus the effect of exercise multiplied by amyloid. In Table 1, we described the parameters used in the system of equations and the values used in the simulations shown in this paper.
Table 1: Parameter values used in simulations
Source: Table generated using Overleaf, 2025
The Tau equation (2) is key since AD shows that Beta-amyloid accumulation initiates phosphorylation of tau protein, which stabilizes the microtubules inside neurons. We use the equation of phosphorylation of tau protein as presented in Hao et al. (2022). In the Neurodegeneration equation (3), the Tau protein induces neurodegeneration. The deposition within cells disrupts microtubules, imparting axonal transport; because of this, P-tau impairs mitochondria and translocates into the nucleus, which causes a progressive loss of nerve cells. Accordingly, we have the equation for N as presented in Hao et al. (2022).
Finally, we have the Cognitive decline equation (4), in which cognitive impairment is determined by tau protein and neurodegeneration, leading to a decline in cognitive function. Therefore, we have the equation for C as presented by Hao et al. (2022) and added two positive terms 𝑏𝐶 and 𝑤𝐶 that represent the addition of the number of Beta-amyloids multiplied by the cognitive element, and added to the effect of exercise with Galantamine treatment on cognition.
Results and Discussion
AD is a neurodegenerative disease that gradually destroys memory, thinking capacity, and the skills to carry out basic functions over time. It is divided into threemain stages. In the early stage, the person retains their independence but experiences notable memory loss. In addition, they may have difficulty performingcomplex tasks. However, in the middle stage, memory loss and confusion intensify, while disorientation and sleep problems arise; the need for support for daily activities increases. In its last stage, the person loses the ability to communicate and control movement; they experience a significant physical decline. AD can be due to age-related changes in the brain, such as shrinkage, inflammation, damage to blood vessels, decreased energy production within the cell, and changes or differences in genes that can be hereditary (Afsar et al., 2023).
There are a variety of treatments used to manage AD. In our research, we specifically studied Lecanemab and Galantamine as pharmacological treatment and physiotherapy as non-pharmacological treatment. Lecanemab is an intravenous antibody that has a high affinity for Beta amyloid plaques. It is a treatment that is approved by the FDA to be applied in the early phase of the disease. If the patient does not within the age range of 50-90 years and the amyloid biomarkers is positive, they are candidate to use the treatments; however, it depends of the medical evaluation (Usc.edu, 2023).The accumulation of plaques in the brain is a pathological process that causes cognitive impairment by disrupting and inhibiting synaptic connections between neurons. Galantamine, on the other hand, is an acetylcholinesterase inhibitor that is used to manage Alzheimer’s disease by increasing acetylcholine levels in the brain to improve memory and cognitive function. Its purpose is to control neuroinflammation and brain metabolic dysfunction, slowing the breakdown of ACh to improve its effect in patients with Alzheimer's, thereby improving their thinking ability (Georgieva et al., 2023). Likewise, physiotherapy is a treatment that involves physical exercise, combined with cognitive activities and tasks that require sequential memory, to help the person manage motor and muscle problems (Chen et. al., 2016).
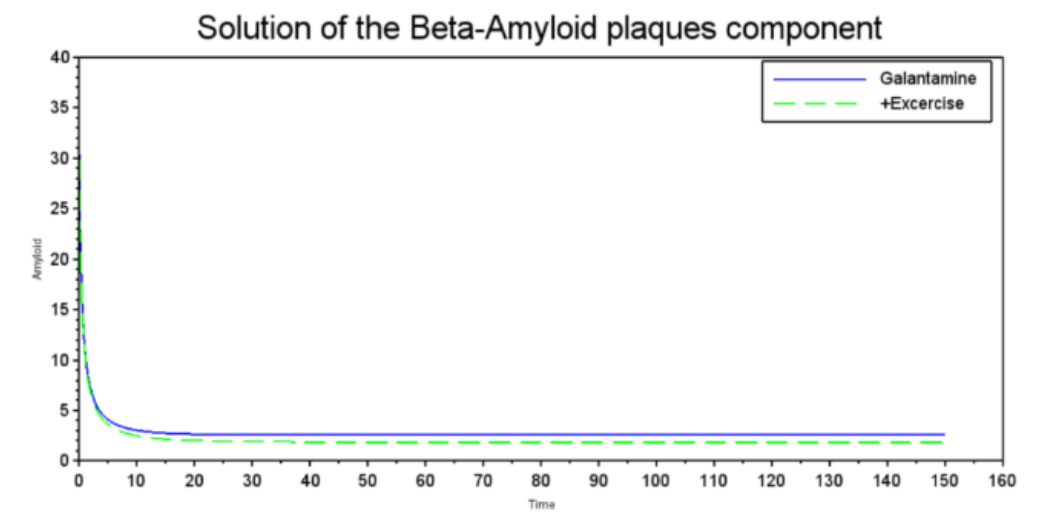
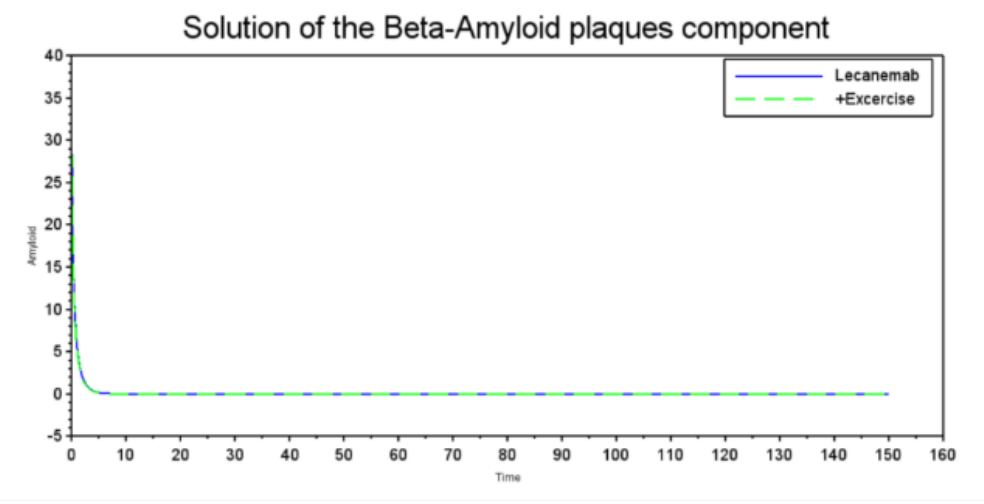
This study employs a mathematical approach to analyze the impact of pharmacological and non-pharmacological treatments on Alzheimer's progression, using differential equations to model biomarkers: tau protein, Beta-amyloid, neurodegeneration, and cognitive decline (Hao et al., 2022). Therefore, the results obtained allow us to systematically analyze the efficiency of interventions such as Lecanemab, Galantamine, and physical exercise. The result emphasizes the development of equations that measure cognition and accumulation of amyloid betas applied in Alzheimer's to reproduce the effects of different pharmacological and non-pharmacological treatments within the disease. Our simulations, shown in Figures 2 to 5, were performed using SCILAB 2024.1.0 using the differential equations shown and the parameter values described in Table 1. The simulations showed that treatments such as Galantamine and physical exercise are effective at reducing beta-amyloid plaque concentration. Figure 2 shows how Galantamine and exercise act on the deterioration or decrease of amyloid plaques as the treatments are used. Galantamine, as an inhibitor of acetylcholinesterase, an enzyme found in the central nervous system, has a direct action on neurodegeneration, while non-pharmacological treatment induces similar effects through physiological mechanisms. In the case of Lecanemab, the monoclonal antibody that reduces the accumulation of plaques, it is almost eliminated compared to physical exercise. The graph compares the effects of Galantamine (blue, solid line) and exercise (green, dashed line) on Beta-Amyloid plaque concentration. Both quickly reduce the concentration from a high baseline. Galantamine appears slightly more effective, stabilizing at a slightly lower level than exercise. This suggests that both pharmacotherapy and exercise could be beneficial in reducing beta-amyloid plaques, a relevant factor in Alzheimer's disease.
Figure 2: Solution of Beta-Amyloid plaques component
Source: Graph generated using our computational model run using scilab 2024.1.0, 2024
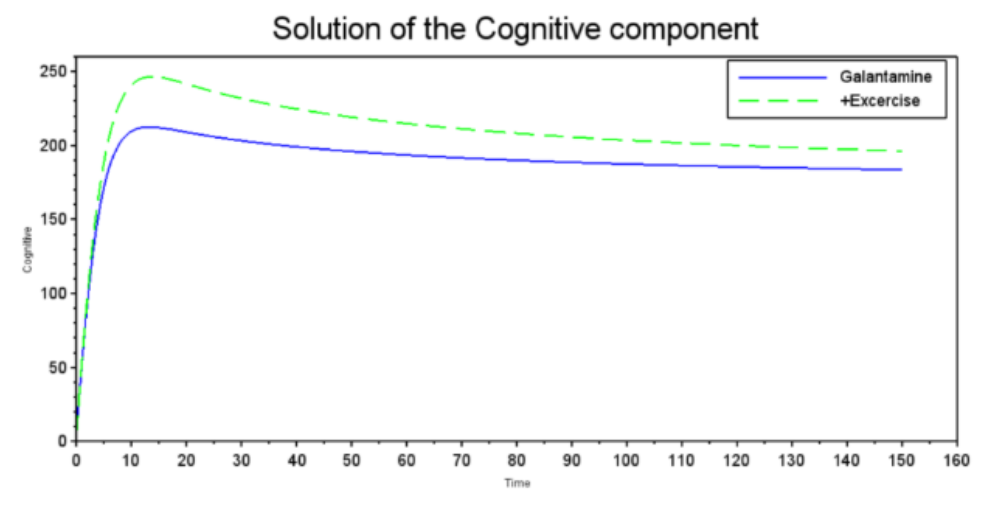
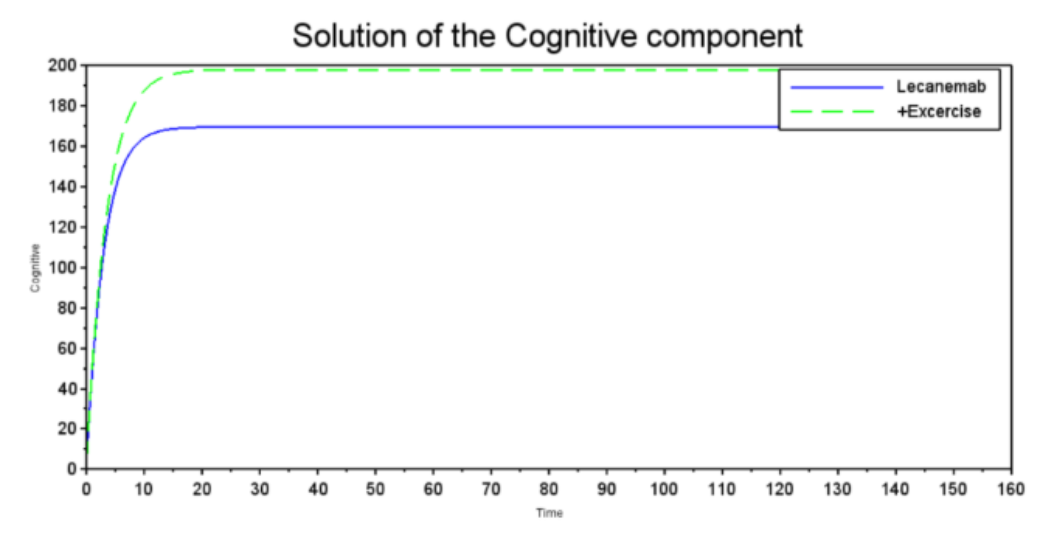
Figure 3 shows the variation in cognitive function over time, demonstrating that both treatments generate a benefit. However, the green dashed curve stabilizes at a level higher than the blue solid curve, suggesting that there is a potential and beneficial exercise-based enhancement of Galantamine. The graph compares the effect of Galantamine (blue solid line) and Galantamine plus exercise (green dashed line) on a cognitive component. Both rapidly improve cognitive function initially, with exercise reaching a higher peak. Although both show a slight decline from the peak, exercise stabilizes at a consistently higher cognitive level than Galantamine in this model over the long term. In Figure 4, Lecanemab and exercise show an almost complete reduction of amyloid plaques, suggesting high efficacy in removing Beta-Amyloid plaques, a key pathological factor in Alzheimer's disease The graph shows the concentration of Beta-Amyloid plaques under treatment with Lecanemab (blue solid line) and Lecanemab plus exercise (green dashed line). Both achieve a very rapid, almost complete reduction of plaques from a high baseline to near-zero levels in a short period of time, suggesting similar efficacy to Figure 2 in this model for eliminating Beta-Amyloid plaques, a key factor in Alzheimer's disease From Figure 5, it appears that Lecanemab treatment has an immediate positive effect on cognitive performance; however, it is similar to the Galantamine graph because of the greater effect of exercise on cognitive performance. This result suggests that when both treatments are combined, exercise enhances the effects of Lecanemab and contributes to improvements in cognitive function. The graph shows the evolution of the cognitive component under treatment with Lecanemab (blue solid line) and Lecanemab plus exercise (green dashed line). Both rapidly improve the cognitive component from a low baseline. Exercise plus Lecanemab reaches a slightly higher final level and stabilizes at a higher value than Lecanemab, suggesting that, based on this model, exercise with Lecanemab might be marginally more beneficial for this cognitive component in the long term.
Figure 3: Solution of the Cognitive component
Source: Graph generated using our computational model run using Scilab 2024.1.0, 2024
Figure 4: Solution of the Beta-Amyloid plaques component
Source: Graph generated using our computational model run using Scilab 2024.1.0, 2024
Figure 5: Solution of the Cognitive component
Source: Graph generated using our computational model run using Scilab 2024.1.0, 2024
In terms of cognitive function, the results of combining exercise with pharmacological treatment indicate that exercise produces a sustained improvement compared with pharmacological treatment alone. However, although drugs show significant improvement on their own, exercise plus medications maintains higher cognitive levels. This relates to previous studies that point out the role of neurogenesis in the formation of neurons in the brain, validating the potentiating effect of combined non-pharmacological and pharmacological treatments in Alzheimer's (Delgado-Peraza et al., 2023).
By integrating pharmacological and non-pharmacological treatments, we are highlighting the importance of a therapeutic strategy. In other words, they complementeach other, while drugs act on biochemical processes, such as accumulation of Beta-amyloids and Tau protein, the physical element influences physiologicalprocesses, such as increased blood flow, therefore improving the patient's quality of life. However, it is important to recognize that the mathematical model has limitations. The simplification of the biological processes summarized in the differential equations omits variables, such as the diversity in DNA composition, which can influence the response to treatment. Likewise, the model's limitations provide us with tools to explore and investigate different therapeutic scenarios that may help delay the progression of Alzheimer's.
The findings of this study highlight the importance of considering novel approaches that combine pharmacological and non-pharmacological treatments for AD. Using differential equation-based mathematical models, it is possible to evaluate the relationship between different pharmacological and non-pharmacological treatments, such as Galantamine and exercise, before patients use them. Evidence and simulations suggest that exercise plays a fundamental role in slowing down Alzheimer's disease and improving cognitive function, demonstrating that physiological and pharmacological treatments in Alzheimer's provide a positive outcome in Alzheimer's disease progression.
Conclusion
This research evaluated different treatments that can help delay Alzheimer's disease at an early stage. Lecanemab showed promising results in reducing Beta-amyloid plaques in the brain, although its effect on cognitive function was limited. Likewise, when Galantamine and physiotherapy were evaluated, it was shown that there would be improvement in the patient's quality of life. The mathematical model provided important insights into how proteins relate and interact, as well as their responses to treatments. Other limitations include the cognitive effects of exercise, which we know exist. However, there are no studies that quantify this improvement as of the writing of this, so we are estimating the effect of this parameter on the model.
Future work
In future work, an important aspect of the deeper study of the disease is to examine the effects of neurodegeneration, in addition to the effects of tau proteins on the mathematical model. The accumulation of tau proteins will cause the development of tangles that induce neuronal death and lead to neurodegeneration. More studies should be conducted to quantify the effects of exercise on cognition. To investigate how physical activity can decrease neurodegenerative processes in order to establish model parameters and understand how non-pharmacological treatments can alter the course of Alzheimer’s. Finally, we must supplement studies of pharmacological and non-pharmacological treatments to expand the range of available treatments.
Acknowledgements
Thanks to Dr. Mayteé Cruz Aponte for the opportunity to work in her Biomathematics research Lab at UPR Cayey and for being our research mentor.
References
Afsar, A., Chacon Castro, M. D. C., Soladogun, A. S., & Zhang, L. (2023). Recent development in the understanding of molecular and cellular mechanisms underlying the etiopathogenesis of Alzheimer’s disease. International Journal of Molecular Sciences, 24(8), 7258.https://pubmed.ncbi.nlm.nih.gov/37108421/
Alzheimer’s Association. (2025). 2025 Alzheimer’s disease facts and figures. Alzheimers Dement 2025, 21(5). Retrieved 11/01/2025 from, https://www.alz.org/getmedia/ef8f48f9-ad36-48ea-87f9-b74034635c1e/alzheimers-facts-and-figures.pdf
Alzheimer’s Therapeutic Research Institute. (2023, April 13). Treating Alzheimer’s with lecanemab: A clinical guide. Usc.edu. https://atrinews.usc.edu/resources/treating-alzheimers-with-lecanemab-a-clinical-guide
Chen, W. W., Zhang, X., & Huang, W. J. (2016). Role of physical exercise in Alzheimer's disease. Biomedical Reports, 4(4), 403–407.
Copilot. (2025). Alzheimer’s disease progression: Amyloid Beta to Cognitive Decline [AI-generated image]. Microsoft Copilot. https://copilot.microsoft.com
Delgado-Peraza, F., Nogueras-Ortiz, C., Simonsen, A. H., Knight, D. L. D., Yao, P. J., Goetzl, E. J., ... & Kapogiannis, D. (2023). Neuron-derived extracellular vesicles in blood reveal effects of exercise in Alzheimer’s disease. Alzheimer's Research & Therapy, 15(1), 156. https://pubmed.ncbi.nlm.nih.gov/37730689/
Departamento de Salud. (2025, 25 de septiembre). Salud lanza nuevo Plan Estratégico 2026-2030 para atender el Alzheimer y las demencias relacionadas en Puerto Rico. Retrieved 11/1/2025 from https://www.salud.pr.gov/CMS/677
Hao, W., Lenhart, S., & Petrella, J. R. (2022). Optimal anti-amyloid-beta therapy for Alzheimer’s disease via a personalized mathematical model. PLoSComputational Biology, 18(9), e1010481.
Mayo Clinic. (n.d.) Enfermedad de Alzheimer. Retrieved 11/01/2025 from e https://www.mayoclinic.org/es/diseases-conditions/alzheimers-disease/symptoms-causes/syc-20350447
Raskind, M. A., Peskind, E. R., Truyen, L., Kershaw, P., & Damaraju, C. V. (2004). The cognitive benefits of galantamine are sustained for at least 36 months: a long-term extension trial. Archives of Neurology, 61(2), 252–256.
Salabert, E. (2023, May 5). Lecanemab, el nuevo fármaco que frena el alzhéimer: dudas y certezas. WebConsultas.https://www.webconsultas.com/noticias/medicamentos/lecanemab-el-nuevo-farmaco-que-frena-el-alzheimer-dudas-y-certezas
Scilab. (2019). Scilab.org. https://www.scilab.org/
Esta obra está bajo una licencia de Creative Commons Reconocimiento-NoComercial 4.0 Internacional.
![[in]genios](http://images.squarespace-cdn.com/content/v1/51c861c1e4b0fb70e38c0a8a/48d2f465-eaf4-4dbc-a7ce-9e75312d5b47/logo+final+%28blanco+y+rojo%29+crop.png?format=1500w)